


ARGUS Umweltbiotechnologie GmbH



© 2024 ARGUS Umweltbiotechnologie GmbH

Quality management
Fundamentals
All works are directed by a qualified Supervisor. The supervisor is announced by name before the commencement of works. The works are carried out by our own specifically and technically qualified staff, whose qualifications are regularly up-dated and developed in line with continued training.
All work areas within ARGUS GmbH are integrated within the Quality Management System (QMS), such as Sampling, Environmental Analysis and Remediation. All employees are aware of the general controls within the QMS. A close working relationship between the project manager, field assistant and the laboratory provides quality and ongoing improvements of works.
The technical competence of our departments, the condition of our equipment and the standard of our works are appraised through internal and external audits as well as through organization, documentation and filing of legal specifications. Current standard and law changes are promptly incorporated into our QMS.
We work closely with our clients and government departments at all stages of the project development. In this way we receive continuous feedback on satisfaction of our clients and government departments regarding performance and service and can therefore react fast to their requests.
Before commencement of works, participating staff are briefed on the danger and contact/handling of hazardous substances to be used on the project through inquiry and operational induction e.g. hazardous substances ordinance. Employees participate in occupational health check-ups. Special considerations which apply to all practices are BGR 128, TRGS 524, UVV und die BaustellV.
Successful carrying-out of sampling, laboratory analysis and remediation is achieved through the capability of the technique and consideration of the applicable standards and regulations. All testing and measuring equipment used in the works belong to ARGUS GmbH and are subject to documented calibration and maintenance plans.
All work areas within ARGUS GmbH are integrated within the Quality Management System (QMS), such as Sampling, Environmental Analysis and Remediation. All employees are aware of the general controls within the QMS. A close working relationship between the project manager, field assistant and the laboratory provides quality and ongoing improvements of works.
The technical competence of our departments, the condition of our equipment and the standard of our works are appraised through internal and external audits as well as through organization, documentation and filing of legal specifications. Current standard and law changes are promptly incorporated into our QMS.
We work closely with our clients and government departments at all stages of the project development. In this way we receive continuous feedback on satisfaction of our clients and government departments regarding performance and service and can therefore react fast to their requests.
Before commencement of works, participating staff are briefed on the danger and contact/handling of hazardous substances to be used on the project through inquiry and operational induction e.g. hazardous substances ordinance. Employees participate in occupational health check-ups. Special considerations which apply to all practices are BGR 128, TRGS 524, UVV und die BaustellV.
Successful carrying-out of sampling, laboratory analysis and remediation is achieved through the capability of the technique and consideration of the applicable standards and regulations. All testing and measuring equipment used in the works belong to ARGUS GmbH and are subject to documented calibration and maintenance plans.
Quality management: sampling
Quality management: sampling
Quality management: sampling
Quality management: sampling
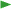
Quality management: laboratory
Quality management: laboratory
Quality management: laboratory
Quality management: laboratory
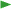
Quality management: remediation
Quality management: remediation
Quality management: remediation
Quality management: remediation
The accreditation is valid for
the test methods listed in the annex to the certificate.
the test methods listed in the annex to the certificate.
DAkkS accredited in accordance with DIN EN ISO/IEC 17025:2018

The accreditation is valid since: Juli 23, 2024


Annex to the accreditation certificate
A
Annex to the accreditation certificate
A
Annex to the accreditation certificate
A
List of accredited procedures in the flexible accreditation area
A
List of accredited procedures in the flexible accreditation area
A
List of accredited procedures in the flexible accreditation area
A
Downloads:


